Prescription Drug Labels: Actions Needed to Increase Awareness of Best Practices for Accessible Labels Blind or Visually Impaired
What GAO Found
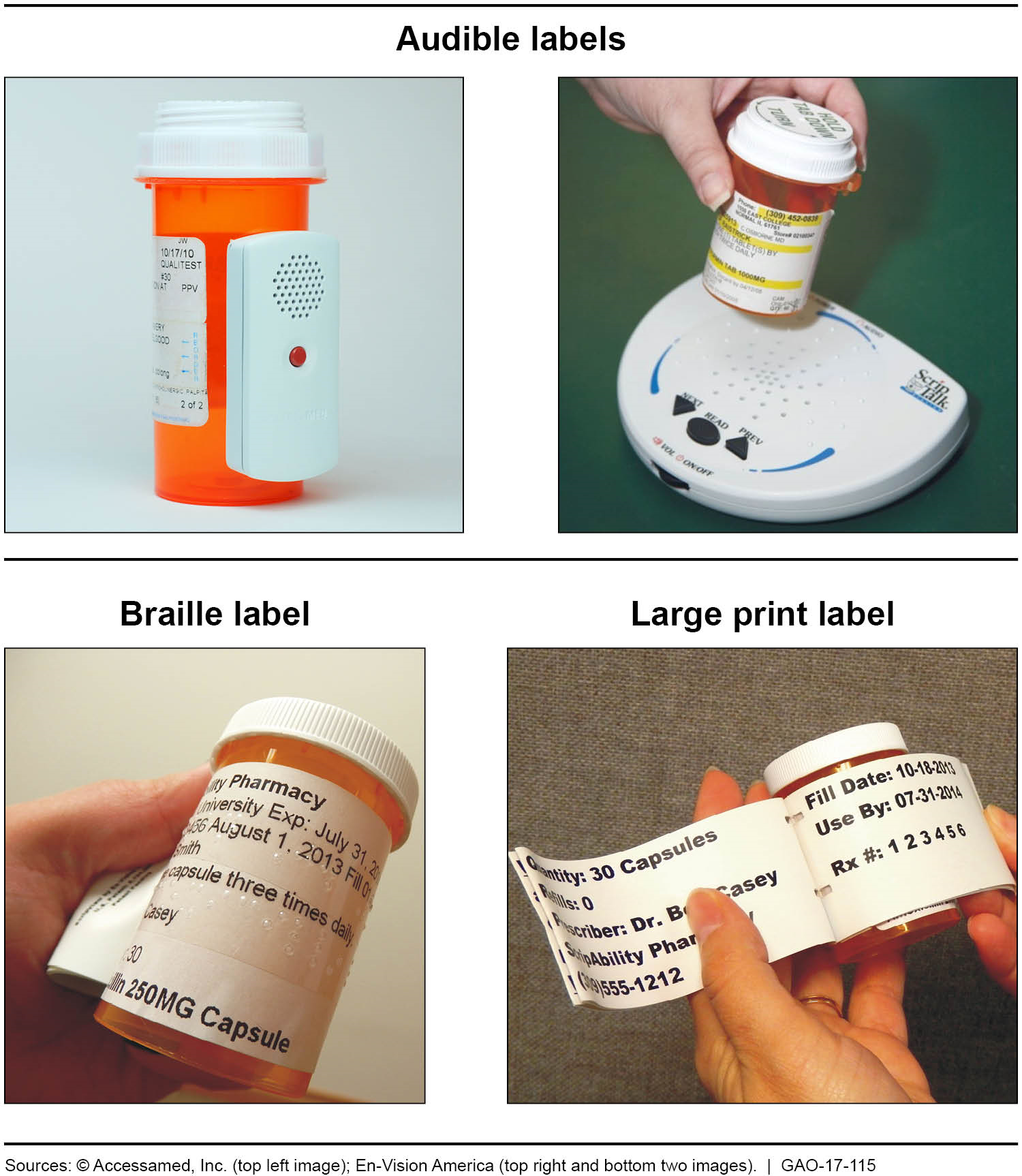
GAO found that some pharmacies can provide accessible prescription drug labels, which include labels in audible, braille, and large print formats and are affixed to prescription drug containers.
Mail order pharmacies: Four pharmacy benefit managers (PBMs) used by large insurers that GAO contacted reported that they can provide accessible labels through their mail order pharmacies.
Retail pharmacies: Six of the 9 largest chain pharmacy companies and 8 of the 18 selected individual retail pharmacy locations GAO contacted also reported that they can provide accessible labels through their store-based retail pharmacies.
The percent of prescriptions dispensed with accessible labels was generally low — less than one percent of all prescriptions dispensed—according to some PBMs and chain pharmacy companies that GAO contacted. With regard to best practices, a working group convened by the US Access Board — a federal agency that promotes accessibility for individuals with disabilities — developed and published 34 best practices for accessible labels. Four PBMs, six chain pharmacy companies, and eight individual retail pharmacy locations GAO contacted reported that they have generally implemented most of the 34 best practices for accessible labels. However, stakeholders GAO contacted said that individuals who are blind or visually impaired continue to face barriers accessing drug label information, including identifying pharmacies that can provide accessible labels.
Stakeholders GAO contacted identified four key challenges that pharmacies faced in providing accessible labels or implementing the best practices: (1) lack of awareness of the best practices; (2) low demand and high costs for providing accessible labels; (3) technical challenges for providing these labels; and (4) an absence of requirements to implement the best practices. Many stakeholders identified greater dissemination of the best practices as a step, among others, that could help address some of these challenges.
The National Council on Disability (NCD) — the federal agency responsible for conducting an informational campaign on the best practices, as required by the Food and Drug Administration Safety and Innovation Act (FDASIA) — has conducted limited campaign activities. Primarily in 2013 and 2014, NCD used its website and social media to disseminate an agency statement and press releases on the best practices. However, most stakeholders GAO spoke with said they had no communication with NCD about its campaign, and some said they were unaware of the best practices. Agency officials provided GAO with an original plan for conducting campaign activities through 2014, but most activities were not conducted. During the course of our review, NCD developed a corrective action plan for conducting future campaign activities. However, neither plan assigned responsibilities for conducting these activities nor does the agency have plans to evaluate them, which is inconsistent with federal internal control standards. Without assigning responsibilities and developing an evaluation plan, NCD will be unable to adjust its action plan and assess whether the information on the best practices is effectively reaching its target audience.
Why GAO Did This Study
About 7.4 million Americans are blind or visually impaired and may face difficulty reading prescription drug container labels. FDASIA required the US Access Board to develop best practices for accessible labels and NCD to conduct an informational campaign on these best practices.
FDASIA also included a provision for GAO to review pharmacies' implementation of these best practices. This report examines: the extent to which pharmacies can and do provide accessible labels and implement the best practices; pharmacy challenges; and the extent to which NCD conducted its informational campaign, among others.
GAO collected information from 55 stakeholders, including 4 PBMs used by large insurers; 9 of the largest chain pharmacy companies; 18 randomly selected individual retail pharmacy locations in 4 states with varying levels of visually impaired residents; and 24 others, such as state regulating bodies and advocacy and industry groups. GAO sent a web-based questionnaire to PBMs, chain pharmacy companies, and individual retail pharmacy locations. GAO also interviewed stakeholders and reviewed state regulations and documents from NCD.
What GAO Recommends
NCD should assign responsibility for conducting campaign activities and evaluate these activities. NCD neither agreed nor disagreed with the recommendation but indicated that it is taking steps to address this issue.
For more information, contact John Dicken at (202) 512-7114 or dickenj@gao.gov.
More Articles
- A Yale Medicine Doctor Explains How Naloxone, a Medication That Reverses an Opioid Overdose, Works
- KFF, Kaiser Family Foundation: Vaccinating Children Ages 5-11; Policy Considerations for COVID-19 Vaccine Rollout
- Pharmacy Gag Rule: There Might Be a Cheaper Drug, But Pharmacists Can’t Tell You That
- That First Job, That Part-time Job, That Retirement Job: The changing face of retail trade
- And That's the Way It Is